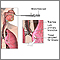
Non-small cell lung cancer
Highlights
Risk:
- Smoking appears to be the primary cause of 85 - 90% of lung cancers. The latest evidence suggests that newly diagnosed patients with early stage lung cancer who quit smoking can significantly improve their outcomes.
- It is estimated that patients with early stage cancer who quit smoking have a 70% chance of survival, compared to 33% in those who continue to smoke.
- Radiographic screening to detect lung cancer in asymptomatic patients at high risk has been debated and studied for several years. Although annual low-dose CT scans have shown benefit in current and former smokers, widespread screening is not yet recommended for asymptomatic patients. Large trials are under way.
Treatment:
- Staging is an important part of a patient's treatment plan. After a rigorous review of current data, the International Association for the Study of Lung Cancer (IASLC) has revised the lung cancer staging system. Additional delineations in tumor size and other descriptors were added to the non-small cell lung cancer staging system.
- Several surgical options are available for various stages of lung cancer. Experts are looking at newer risk profiles with an aim toward doctor-patient decision making rather than strict eligibility rules. Patients may or may not accept the risks of surgery along with the possible benefits.
- Video-assisted thoracoscopic surgery (VATS) is becoming more widely used to treat early stage lung cancer patients. While the less invasive option was initially considered for wedge resections, it is now also used for more complex lobectomies and segementectomies.
- Certain tumors respond differently to specific drug therapy options. Research is now focused on protein biomarkers and gene mutations that give rise to the tumor. With this information, caregivers can match customized chemotherapy and other drug treatments with for advanced NSCLC patients.
- Gefitinib (Iressa), erlotinib (Tarceva), and cetuximab (Erbitux) are among the newer agents being used for treatment based on this type of testing.
- For patients with advanced stage non-small cell lung cancer, a palliative care program started at the time of diagnosis and continued throughout the continuum of care (combined with standard cancer treatments) has shown to improve survival, quality of life, and mood.
Introduction
Although lung cancer accounts for only 15% of all newly-diagnosed cancers in the United States, it is the leading cause of cancer death in U.S. men and women. It is more deadly than colon, breast, and prostate cancers combined. About 160,000 patients die from lung cancer each year. Death rates have been declining in men over the past decade, and they have about stabilized in women.
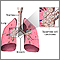
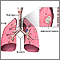
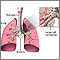
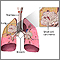
The Lungs
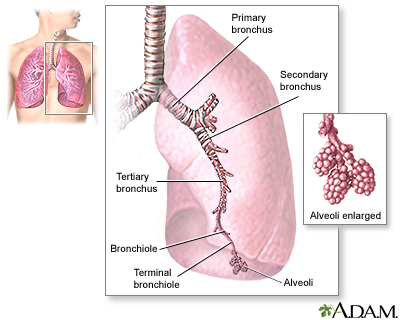
The lungs are two spongy organs surrounded by a thin moist membrane called the pleura. Each lung is composed of smooth, shiny lobes: the right lung has three lobes, and the left has two. About 90% of the lung is filled with air. Only 10% is solid tissue.
- Air is carried from the trachea (the windpipe) into the lung through flexible airways called bronchi.
- Like the branches of a tree, the bronchi in turn divide into over a million smaller airways called bronchioles.
- The bronchioles lead to grape-like clusters of microscopic sacs called alveoli.
- In each adult lung, there are about 300 million of these tiny alveoli. A thin membrane makes up the alveoli sacs. Oxygen and carbon dioxide pass through this membrane to and from capillaries.
- Capillaries, the smallest of our blood vessels, carry blood throughout the body.
Lung Cancer
Lung cancer develops when genetic mutations (changes) occur in a normal cell within the lung. As a result, the cell becomes abnormal in shape and behavior, and reproduces endlessly. The abnormal cells form a tumor that, if not surgically removed, invades neighboring blood vessels and lymph nodes and spreads to nearby sites. Eventually, the cancer can spread (metastasize) to locations throughout the body.
The two major categories of lung cancer are small cell lung cancer and non-small cell lung cancer. Most lung cancers are non-small cell cancer, the subject of this report. Less common cancers of the lung are known as carcinoids, cylindromas, and certain sarcomas (cancer in soft tissues). Some experts believe all primary lung cancers come from a single common cancerous (malignant) stem cell. As it copies itself, that stem cell can develop into any one of these cancer types in different people.
In addition, cancers in the lung may have spread from other sites, such as the breast, thyroid, or colon. In these cases, doctors name the cancer after its original location, such as "breast cancer with lung metastases."
Non-Small Cell Lung Cancers
Non-small cell lung cancers are categorized into three types:
- Squamous cell carcinoma (also called epidermoid carcinoma)
- Adenocarcinoma
- Large cell carcinoma
These separate types are grouped together because, in the early stages before the cancers have spread, they all can be treated with surgery.
Squamous Cell Carcinoma. Squamous cells are formed from reserve cells. These are round cells that replace injured or damaged cells in the lining (the epithelium) of the bronchi, the major airways. Tumors formed from squamous cells are usually found in the center of the lung, either in a major lobe or in one of the main airway branches. They may grow to large sizes and form cavities in the lungs.
When squamous cell cancer spreads, it may travel to the bone, adrenal glands, liver, small intestine, and brain.
Squamous cell carcinoma is nearly always caused by smoking, and it used to be the most common cancer. It still makes up 25 - 30% of all lung cancers.
Adenocarcinoma. Adenocarcinomas usually start from the mucus-producing cells in the lung. About two-thirds of adenocarcinomas develop in the outer regions of the lung, while one-third develop in the center of the lung.
In 1965, 12% of lung cancers were adenocarcinomas. They are now estimated to account for 40% of all lung cancers and are the most common lung cancers in many countries. They are also the most common lung cancers in women, and their rates are increasing dramatically in men. Until recently, adenocarcinoma was only weakly linked to smoking. Experts now suggest, however, that the dramatic increase in this lung cancer type in recent decades may be due to low-tar, filtered cigarettes. People who smoke them draw tiny particles deeper into their lungs.
The course of this cancer varies widely. Most often, it develops slowly and causes few or no symptoms until it is far advanced. In some cases, however, it can be extremely aggressive and rapidly fatal. In 50% of cases in which this cancer spreads, it spreads only to the brain. It also can spread to the other lung, liver, adrenal glands, and bone.
Bronchoalveolar Lung Cancer. Bronchoalveolar lung cancer is actually a subtype of adenocarcinoma. It develops as a layer of column-like cells on the lung and spreads through the airways, causing great volumes of sputum. This cancer also is increasing in incidence.
Large Cell Carcinoma. Large cell carcinoma, which makes up about 10 - 15% of lung cancers, includes cancers that cannot be identified under the microscope as squamous cell cancers or adenocarcinomas.
Small Cell Lung Cancer
Small cell lung cancer may, like squamous cells, originate from reserve cells or other cells in the epithelium. It causes 10 - 15% of all lung cancers. Without chemotherapy, it is very aggressive and usually rapidly fatal. It requires a different treatment approach from non-small cell lung cancer, so it is not discussed in this report.
Causes
Cigarette Smoke. Smoking causes 87% of lung cancer deaths, and accounts for 30% of all cancer-related deaths. Cigarettes, nicotine, or both may contribute to lung cancer in one or more of the following ways:
- The smoke is the most dangerous component of the cigarette. Chemicals formed during smoking trigger genetic mutations that lead to cancer. When people inhale cigarette smoke, they bring into their lungs tar that includes over 4,000 chemicals, some of which are carcinogenic (cancer-causing). Other inhaled chemicals in cigarette smoke that may increase the risk for cancer include cyanide, benzene, formaldehyde, methanol (wood alcohol), acetylene (the fuel used in torches), and ammonia. Smoke also contains nitrogen oxide and carbon monoxide, both harmful gases.
- Nicotine is responsible for the addicting properties of tobacco. It is not clear if nicotine itself plays a role in the growth of cancer cells. In any case, nicotine replacement products are much, much safer than tobacco.
Radon. Radon is a gas produced naturally by the breakdown of uranium. It is often present in the soil and in water and can seep into any dwelling. Radon is the second leading cause of lung cancer.
Other Contributors. Toxic particles leading to precancerous changes in the lung are also found in marijuana. Multiple studies report an association between abnormal lung changes and marijuana smoking.
There is considerable debate over the lung cancer risk posed by depleted uranium used in military weapons (such as in the Gulf and Balkan conflicts).
Other lung carcinogens include asbestos, arsenic, certain petrochemicals (materials made from crude oil or natural gas), and other airborne (carried through the air) byproducts of various mining and manufacturing processes.
Genetic Mutations
Genetic mutations that cause cancer generally occur in two types of genes:
- Tumor-suppressor genes, which prevent cells from endlessly copying themselves
- Proto-oncogenes, which encourage cells to keep making copies of themselves [when a proto-oncogene changes (becomes mutated), it is then called an oncogene]
Damage to either type of gene can cause a mutation that results in uncontrolled division of cells. This uncontrolled division forms tumors.
It is unlikely that a single specific abnormality causes all lung cancer. It probably takes a variety of mutations to start the devastating chain of events leading to cancer. The following mutations are among those under investigation:
- EGFR mutations: EGFR (epidermal growth factor receptor gene) is a family of genes that can mutate and promote tumor growth. This gene mutation is often implicated in non-smokers. HER2 is a related gene under study that plays a role in regulating cell growth.
- BPDE-caused mutations: The chemical BPDE, a byproduct of tobacco smoke, is involved with a number of genetic mutations, including those to an oncogene called K-ras and to three tumor-suppressor genes known as p53, PPP2R1B, and p16. (Tumors that contain the p53 mutation may also be more resistant to chemotherapy.)
- Rb mutations: Another important contributor to lung cancer is a genetically defective protein called retinoblastoma (Rb), which is associated with very aggressive tumors. Low levels of the normal Rb gene may sometimes predict aggressive cancer, especially in patients with small cell lung cancer.
- Abnormalities in the FHIT gene: Such abnormalities may cause the cells lining the lung to become more vulnerable to the effects of tobacco smoke and other cancer-causing substances.
- Alpha1-antitrypsin mutations: People who carry a common variation in the gene for alpha1-antitrypsin -- a substance that normally protects the walls of the alveoli in the lungs -- are 70% more likely to develop lung cancer than those without the mutation, regardless of whether they smoke.
- Many other gene mutations have been implicated including BRCA1,RAP80, RRM1, ERCC1, and TS. Scientists continue to explore the complex relationship between various genes that play a role in cell production and what environmental factors give rise to cancer.
- Medical centers are beginning to test tumors for specific gene mutations affecting tumor growth. The hope is that an accurate "genetic fingerprint" can help doctors prescribe the most effective and appropriate treatment options.
Symptoms
Lung cancer is unlikely to produce symptoms until the disease is advanced. When symptoms develop, they may result from the lung tumor itself, from its effects on tissues outside the lung, or from the spread of cancerous cells to other organs.
Initial symptoms
The first symptoms of lung cancer may include some of the following:
- Frequent bouts of pneumonia, or pneumonia that does not clear up in a normal period of time
- Coughing that does not go away or coughing up blood
- Weight loss
- Fever
- Shortness of breath
- Chest pain
Symptoms of Later Stages
Later-stage symptoms and complications include the following:
- Shortness of breath: This common symptom is the result of cancer that has spread in the lung and the pleura -- the membrane covering the lung.
- Superior vena cava syndrome: In some cases, tumor growth or spreading of the cancer presses against the superior vena cava, a large vein that returns blood from the upper part of the body to the heart. When this happens, a condition called superior vena cava syndrome may occur, leading to obvious swelling in the arms and face.
- Trouble swallowing: The esophagus is the pipe that takes food from the mouth to the stomach. The cancer may spread to or press against the esophagus, interfering with swallowing and nutrition.
- Hoarseness: Cancer can damage the nerves that control the voice box, causing hoarseness.
- Pancoast syndrome: Damage to the brachial plexus, a group of nerves branching from the neck, can cause pain, weakness, or numbness in the arm or hand (Pancoast syndrome).
- Bronchoalveolar lung cancer may produce very large amounts of mucus.
- Hypercalcemia: Some lung cancers produce substances that remove calcium from bone and release it into the bloodstream, causing a condition called hypercalcemia. Patients with this disorder can experience nausea, vomiting, constipation, weakness, and fatigue.
Other lung cancers (usually small cell cancer) cause the body to retain water, lowering the blood's sodium levels. This condition, called hyponatremia, can produce confusion, weakness, and even seizures.
Risk Factors
Before cigarettes became popular in the beginning of the 20th century, lung cancer was rare. It now strikes about 226,000 Americans per year, and some 160,000 die from it annually. The disease usually occurs in people over 50 years old. Men have a significantly greater incidence of lung cancer compared to women. On the encouraging side, the rate of lung cancer in men has been declining significantly over the past decade. While lung cancer rates have been increasing dramatically in women (by 600% from 1950 to 2000), they now appear to be stabilizing. However, lung cancer deaths among female nonsmokers seem to be on the rise.
Smokers and Those Exposed to Cigarette Smoke
Smoking is the primary cause of 85 - 90% of lung cancers. The risk of lung cancer in smokers is about 20 times that of nonsmokers. The risk depends on the duration of the addiction and the number of pack years. (One pack year equals the number of packs of cigarettes smoked per day, multiplied by the number of years that the person has smoked.) Genetic damage in the lung occurs in nearly all chronic smokers, even if cancer has not developed.
People who smoked can be at increased risk for lung cancer more than 20 years after quitting, although the risk drops significantly even in the first year after quitting. There are benefits to quitting smoking, even for people who are well into middle age. Evidence suggests that quitting smoking, after a diagnosis of early stage lung cancer, improves outcomes significantly.
Risk for Lung Cancer by Age 75 According to Quitting Age | |
Quitting Age | Percentage |
30 | 2% |
40 | 3% |
50 | 6% |
60 | 10% |
Secondhand Smoke. The Environmental Protection Agency has classified secondhand smoke as a carcinogen (cancer-causing chemical). Exposure to secondhand tobacco smoke increases the risk of lung cancer in the nonsmoker by about 20 - 30%. A 2006 Surgeon General report found that about 3,000 nonsmokers die each year of lung cancer resulting from exposure to secondhand smoke.
Ethnic Differences
There may be some ethnic differences in lung cancer risk. For example, African-American men have about a 45% higher risk of developing lung cancer than Caucasian men. It is not clear what factors are responsible for this higher risk. Some African-Americans appear to have a genetic vulnerability to the harmful chemicals in cigarette smoke.
In China, about one-third of all young male smokers will eventually die because of tobacco-related illnesses. Their risk for lung cancer, however, is much less than it is for chronic lung disease, the opposite of the Western trend. The lower rate of lung cancer among Chinese people might be due to a slow rate of clearing nicotine, which results in smoking fewer cigarettes.
Socioeconomic Differences
Low income and a lack of education have been linked to an increased risk for lung cancer. Researchers say socioeconomic status is connected to other factors involved in lung cancer risk, such as smoking, diet, and exposure to cancer-causing chemicals in the workplace.
Environmental Factors
People with High Exposure to Radon. Studies have shown that radon raises the risk of lung cancer in underground miners by 40%. It is unclear whether the results of these studies would apply to people exposed to radon in their homes. Homes or buildings built on landfills that contain high levels of radon are the most likely sources of this low level, chronic exposure.
A cumulative long-term exposure to radon and smoking also increases the danger. Most people move an average of 10 or 11 times over their lifetime, so the risk of developing lung cancer through radon exposure is very low in most individuals, even for those who lived for a while in areas with high radon levels. People with homes that have high radon levels and those who sleep or spend a long time in basements with detectable but moderate levels should consider taking protective measures.
Workers Highly Exposed to Carcinogens. An estimated 9,000 - 10,000 men and 900 - 1,900 women develop lung cancer each year because of occupational exposure to carcinogens. More than half of these cases are attributable to past exposure to asbestos, which has long been known to be a risk factor for mesothelioma (cancer of the pleura, the lining around the lung) and can increase the risk of lung cancer in smokers. With better protective measures, these rates are expected to fall in the future.
Other chemicals that put workers at risk for lung cancer include:
- Arsenic (insecticide and herbicide sprayers, tanners, oil refinery workers)
- Chloromethyl methyl ether (workers exposed to certain polymers, water repellents, or products using chloride and formaldehyde)
- Chromium compounds (workers using certain alloys, paints, pigments, and preservatives)
- Depleted uranium (soldiers exposed to weapons during battlefield conditions)
- Crystalline silica
By contrast, agricultural workers seem to have a lower lung cancer rate, despite their possible occupational exposures to risky chemicals. While this rate has traditionally been attributed to good health habits, including low tobacco use, agricultural workers' exposure to endotoxin may be responsible. Endotoxin is a component of common bacteria found in soil and animals, and it may have cancer-preventing effects on the immune system.
Air Pollution. Although any risk from air pollution is very small, it nevertheless may be a contributor to those lung cancers not obviously related to smoking. Some studies have found an association between increased risk for lung cancer and long-term exposure to very small particulates, especially sulfates, in polluted air. The risk, if any, is very small.
Family History
A family history of lung cancer may play a role in increasing susceptibility to this disease. Women who had mothers or sisters with lung cancer have triple the risk. The risk is higher in both smokers and nonsmokers. There is no association between a history of other cancers and lung cancer. Both genetic factors and secondhand smoke appear to contribute to the danger in these individuals.
Hormone Replacement Therapy
Research suggests that postmenopausal women taking combined hormone replacement therapy (estrogen plus progestin) may have a higher risk of death from non-small cell lung cancer than women not taking hormones.
Other Diseases that Increase Risk
Smokers with emphysema or chronic inflammatory lung diseases, such as asthma, are at increased risk for lung cancer. Both smokers and nonsmokers whose lungs are scarred from recurrent lung diseases, such as pneumonia or tuberculosis, are also at increased risk, particularly for bronchoalveolar lung cancer.
Lifestyle Changes
Quitting Smoking
Quitting smoking improves lung function almost immediately. Some evidence suggests that the benefits for the lungs are even more significant for women who quit than for men. Furthermore, quitting, even after a diagnosis of lung cancer, improves your chance of survival significantly. It is estimated that patients with early stage lung cancer who quit smoking have a 70% chance of survival, compared to 33% in those who continue to smoke.
It can take 20 years or longer, particularly in heavy smokers, for the lungs to be restored to full health and the risk for lung cancer to be reduced as low as it is for nonsmokers. Quitting is extremely difficult. There are many smoking cessation programs available that can become part of a patient's overall treatment plan. No one should be discouraged if they relapse. Everyone should keep trying to quit. With continued efforts, many people succeed.
At this time perhaps the most effective method for quitting is a combination of the following:
- Nicotine replacement products that reduce withdrawal symptoms and cravings.
- The antidepressants bupropion (Zyban, Wellbutrin), which reduce emotional effects and cravings associated with withdrawal, and improve abstinence rates.
- The drug varenicline (Chantix), which blocks nicotine receptors in the brain. This medication is very effective, but carries a risk of psychiatric side effects. People taking varenicline, especially those with a history of psychiatric problems, should be closely monitored for changes in mood or behavior. Chantix has been linked to a possible increase in heart problems, but since smoking is a major cause of heart disease, the benefits of using Chantix to quit appear to outweigh the possible risks.
- Professional counseling or support organizations that may, in addition to medication, help people stop smoking.
While people are in the process of quitting (and afterward), they should maintain as healthy a lifestyle as possible.
Dietary Factors
The research on diet and cancer suggests that antioxidants in certain foods may protect against the DNA damage that can lead cells to turn cancerous. It's important to note that, although studies have suggested an association between these factors and cancer risk, no cause-and-effect has been proven. It is also important to note that while the antioxidants in foods may be protective, antioxidant supplements actually increase risk in smokers. That makes a healthy diet even more desirable.
Phytochemicals. Some data suggest that diets rich in fresh fruits and vegetables may protect against lung cancer in both smokers and nonsmokers. Those most studied in relationship to protection from lung cancer include phytoestrogens, flavonoids, and glucosinoids.
Note: Studies on these chemicals are not consistent. It is unlikely that individual phytochemicals offer protection, but rather that any benefit comes from a collection of vitamins and plant chemicals contained in fruits and vegetables. Fruit, especially, appears to be protective.
Fats and Oils. Some studies have indicated that diets high in animal fats increase the risk for lung cancer. Others have suggested some protection against lung cancer comes from cod liver oil, which contains omega-3 fatty acids (found in fatty fish), omega-6 fatty acids (found in flax and in soybean and canola oils), and monounsaturated oils (found in olive and canola oils). However, the ability of these substances to protect against lung cancer remains controversial, and quitting smoking remains the best advice.
Vitamin Supplements. Even in those who eat a healthful diet, smoking reduces the body's levels of a number of vitamins, importantly vitamin C. There is no evidence, however, to support any benefit from taking antioxidant supplements, including vitamins C, E, A, folate, or beta carotene.
In fact, evidence is now suggesting that high doses of vitamin C, vitamin E, and especially beta carotene supplements have harmful effects. The strongest studies on the effects of antioxidant supplements have reported an increase in lung cancer and overall mortality rates among smokers who took beta carotene or vitamin E supplements. This is particularly important information for smokers, who may carry precancerous or cancerous cells for years before developing the disease. The best way to get healthy levels of important nutrients is by eating healthy foods.
Trace Element Supplements. Trace elements such as zinc and selenium have been studied for potential protection against lung cancer without any clear evidence to support their benefits.
Protecting the Home against Radon
People concerned about radon in their home or area can purchase a test approved by the Environmental Protection Agency. One way to remove radon is by installing a soil suction system. It should be noted, however, that home prevention measures rarely reduce radon levels to zero. Simply sleeping by an open window reduces the risk.
Diagnostic Tests
Physical Examination. A detailed physical examination of the whole body is very important to identify or rule out the spread of cancer to other areas, and to determine the patient's general condition. For example, questions about dizziness or headaches can help the doctor determine if the cancer has spread to the brain. Bone or joint pain might suggest that the cancer has spread to the bone. The doctor will also look for head and neck symptoms that might indicate other tumors. Also, the patient's weight loss and ability to function are two very important factors for predicting survival following treatment. Patients who are mobile and have lost less than 10% of their pre-treatment weight tend to have better survival rates.
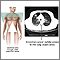
Chest X-Rays. In a small percentage of cases, a routine chest x-ray reveals the first signs of lung cancer. Usually, however, symptoms of existing lung cancer, such as coughing, chest pain, and blood in the sputum, will lead to a chest x-ray. If non-small cell lung cancer is present, chest x-rays may show lesions (damaged or abnormal tissue) in the center of the lung, cavities formed by squamous cell carcinoma, or a lace-like pattern of cells spreading through the lungs. By the time lung cancer is diagnosed by chest x-rays, however, it has often spread so far that it cannot be surgically cured. Four major studies found no survival benefits in early detection from chest x-rays and sputum screening. CT scans have shown to be better than chest x-rays in detecting nodules and lung cancer. Regular screening for lung cancer using x-rays is therefore not recommended.
Computed Tomography. Computed tomography (CT), particularly the specific technique called low-dose spiral (or helical) CT, is more effective than x-rays for detecting cancer in patients with suspected lung cancer. It is the standard imaging procedure for determining if and where the cancer has spread (metastasized). Surgeons also use CT scans to evaluate patients before lung surgery.
The use of helical CT for widespread early screening of asymptomatic patients is currently under debate.
There is evidence that annual CT screening can improve survival in smokers by about 20%. A recent trial showed that current and former smokers who underwent annual CT screening for up to 3 years were less likely to die from lung cancer, or any other cause. Researchers note that the positive effect may be even greater, since the CT scans in use today are even more advanced than those used in the trial between 2002 - 2007.
However, widespread screening for lung cancer is not yet recommended in any population group. Earlier studies on the use of imaging tests for lung cancer screening raised the issues of over-diagnosis, unnecessary invasive testing, and little differences in mortality compared to those that did not undergo screening, but advances in CT technology and recent large trial evidence may alter the landscape. The disadvantages of periodic low-dose CT scans and other aspects of screening are under continued study.
High-risk individuals who are interested in early screening with CT scans should ask their doctor about available clinical trials.
Other Imaging Tests for Staging and Tracking Cancer
Computed tomography is the standard imaging procedure for determining if and where the cancer has spread (metastasized). Other imaging tests, however, may also be useful for staging and tracking lung cancers (staging means finding out how advanced the cancer is).
A bone scan is done to check for spread of cancer to the bones for those with bone pain, or other findings suggesting spread of cancer to the bones.
Positron Emission Tomography. Positron emission tomography (PET), specifically a technique known as FDG-PET can diagnose lung tumors as small as 1 centimeter with very high accuracy. PET works best when used with CT scans.
With this imaging test, the patient is first injected with a specially formulated, radioactive liquid sugar (called FDG), and then viewed with a machine that records energy given off by cells that absorb high levels of FDG, such as lung tumor cells.
PET may be a good imaging technique for staging lung patients thought to have early stage lung cancer after other testing. These patients are considered candidates for surgery intended to cure. But if a PET scan identifies previously undetected spread of the cancer elsewhere in the body, the patient may be able to avoid unnecessary surgery.
However, compared to traditional staging with CT scans, PET-CT may also wrongly diagnosis some with more advanced cancer than they actually have, possibly leading to the incorrect treatment.
Magnetic Resonance Imaging. Magnetic resonance imaging (MRI), an imaging procedure that uses radio wave energy, is frequently used instead of CT scanning to locate brain and bone tumors that have spread from the lung.
Biopsy Procedures
Biopsies of lung tissue are needed to confirm lung cancer. This requires invasive procedures that may vary from simple needle aspiration to chest surgery.
Needle Aspiration. Sometimes, a biopsy specimen is obtained by inserting a needle between the ribs, and then guiding it with the use of CT scans, ultrasound, or fluoroscopy (a device allowing an x-ray view). Specific techniques include transbronchial or transthoracic needle aspiration (TBNA or TTNA), endoscopic ultrasound-guided needle aspiration (EUS-NA) and transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) . Their use depends on how much of the area can be observed with less invasive imaging methods. There is a 5 - 10% risk for bleeding or collapsed lung with needle aspiration.
Thoracoscopy. Thoracoscopy is usually very effective for diagnosing cancer in the outer areas of the lungs, or those involving the pleura (membrane surrounding the lungs). This is a surgical procedure that uses a fiber optic tube to view the area. The procedure requires general anesthesia. The surgeon passes surgical instruments and a fiber optic tube through a small incision in the chest. The tube has a camera in it, which allows the surgeon to look at the lungs on a video screen.
Bronchoscopy. Bronchoscopy can help locate cancer that develops in the central areas and major airways of the lung (usually squamous or small-cell cancer). The procedure is done as follows:
- The patient is given a local anesthetic, oxygen, and sedatives.
- The doctor inserts a bronchoscope -- a hollow flexible tube, often containing a fiber optic light source, into the lower respiratory tract through the nose or mouth.
- The tube acts like a telescope into the body, allowing the doctor to see the windpipe and major airways. In a procedure called fluorescence bronchoscopy, the doctor injects the patient with a drug that makes cancer tissue appear red when exposed to laser light from the bronchoscope.
- The surgeon removes specimens for biopsy, ideally combining techniques to include cutting tissue, brushings, and a washing process called bronchoalveolar lavage (BAL). BAL involves injecting saline through the bronchoscope into the lung and then immediately suctioning the fluid back through the hollow tube of the bronchoscope. The fluid is then analyzed in the laboratory. Both brushing and washing procedures may be very valuable additions to this procedure.
Bronchoscopy is usually very safe, but complications can occur. They include:
- Allergic reactions to the sedatives or anesthetics
- Asthma attacks in susceptible patients
- Bleeding
Patients may develop a fever after the procedure.
Mediastinoscopy. Mediastinoscopy uses a tube inserted in the central part of the lungs to locate the appropriate areas for biopsy. It is performed if the physician suspects that cancer has spread to nearby lymph nodes but has not yet spread to other parts of the body, and to confirm negative biopsy results. This procedure is slowly being replaced by endoscopic and endobronchial methods.
Laboratory Tests
Sputum Analysis for Presence of Cancer Cells. Analysis of coughed-up sputum, performed as a screening test for lung cancer, is often done along with chest x-rays. This method has not reduced death rates. Recent improvements in this screening technique are under study.
Sputum analysis may also be used to diagnose lung cancer in someone with signs of lung cancer. However, it is not 100% accurate. If a sputum analysis does not show cancer cells, other tests are performed.
Biomarkers. Biologic markers, called biomarkers, are high levels of substances that are released by tumors and indicate the presence of specific cancers. Biomarkers can be found in sputum, blood, and tissue samples. They can include:
- Proteins
- Enzymes
- Hormones
- Amino-acid compounds
- Antigens (identified by antibodies that specifically target them)
- Growth factors
- Other chemicals
A number of these biomarkers are being evaluated alone or in combination as either screening tools or as potential markers for the risk of disease progression. Some of these biomarkers may be detectable 1 - 3 years before a clinical diagnosis of lung cancer. The potential for this early diagnosis could mean an improvement in lung cancer survival in
the future.
Other Tests
As part of the doctor's initial examination, patients may have a pulmonary function test and breath analysis to evaluate lung health and capacity. The doctor may also take a complete history of the heart and lungs, because they are often involved in complications following lung cancer surgery.
Staging Systems
In lung cancer, the stage of the disease at the time of diagnosis is a major factor in determining how to treat the cancer, and how long the patient can expect to live. In general, survival is longest for patients with very early-stage disease and shortest for patients with very advanced disease that has spread to several areas of the body. However, some groupings with very different clinical features can have similar prognosis. Staging is based on the results of physical and surgical examinations, and laboratory and imaging tests, including biopsies. A combined approach is necessary for accurate staging. Research has shown that endosonography plus surgical staging produced more accurate staging results than surgical staging alone.
To determine the stage, medical professionals first categorize each tumor by size and by how far it has extended. This identification method is called the TNM system.
The TNM categories then determine the stage (numbered 0 to IV) of the cancer.
The International Association for the Study of Lung Cancer recently revised the non-small cell lung cancer staging system in 2009. Extensive analysis was performed on an international database. The major staging categories remain the same, while additional subgroupings within the T, N, and M descriptors were added.
The TNM System
TNM stands for Tumor, regional lymph Nodes, and Metastasis (cancer spread beyond the original tumor).
T refers to the size and spread of the tumor. In TX and T0, the tumor is either unable to be assessed or indicated by cancer cells in sputum or lung samples but it cannot be seen.
Tis: Carcinoma in situ. The cells are cancerous, but the tumor does not show evidence of spreading.
In T1, the tumor is 3 cm or less in size, is still contained in the lung or the membrane covering the lung, and has not reached the main airway. In T1a, the tumor is less than or equal to 2 cm and in T1b, the tumor is greater than 2 cm but less than or equal to 3 cm in diameter.
In T2, the tumor has one or more of the following features:
- It is greater than 3 cm or less than 7 cm
- It involves the main airway
- It is 2 cm or more away from the ridge (the carina) at the lowest part of the windpipe
- It has invaded the pleura
- It is associated with collapsed lung tissue (atelectasis) or swelling that blocks part (but not all) of the lung
T2 is further broken down in the new staging system. T2a refers to a tumor greater than 3 cm but less than or equal to 5 cm in diameter. T2b is greater than 5 cm but less than or equal to 7 cm.
In T3, a tumor is greater than 7 cm or has directly invaded any of the following:
- Chest wall
- Diaphragm
- Membrane covering organs and structures in the chest
- Outer wall of the membrane around the heart (pericardium)
In addition, one or more of the following conditions are present:
- The tumor is in the main airway, less than 2 cm away from the carina, but is not in the trachea (windpipe).
- The tumor is associated with a collapsed lung or swelling that blocks the entire lung.
In T4, the tumor has invaded any of the following:
- Area between the lungs (mediastinum)
- Heart
- Great vessels (the blood vessels that carry blood from the heart)
- Carina, trachea, or esophagus
- Main portion of the spine
In addition, one or both of the following occurs:
- Separate tumors are present in the same lobe
- The tumor is accompanied by an increased amount of fluid between the pleural membrane and the lung.
N followed by a number from 0 to 3 refers to whether the cancer has reached regional (in the area of tumor) lymph nodes.
- In stage NX, the regional lymph nodes cannot be assessed.
- In stage N0, the regional lymph nodes are still cancer-free.
- In N1, the cancer has spread to the nearest lymph nodes around the airways, to the hilum (a central zone in the lung where blood and lymph vessels enter), or both. The tumor has extended directly into lymph nodes within the lung.
- In N2, the cancer has spread to lymph nodes in the middle of the chest next to the affected lung, to the nodes below the carina, or to both regions.
- In N3 the cancer has spread to lymph nodes in the middle of the chest that are next to the opposite lung, to the hilum in the opposite lung, to lymph nodes in nearby or opposite muscle tissue, or to lymph nodes above the collar bone.
M Stages refer to cancer spread (metastasis).
- In M0, spread has not occurred.
- In M1a, tumor nodules are present in the other lung or on the pleura (the sac surrounding the lungs), or a malignant pleural effusion (cancer cells in the fluid within the pleura) is present.
- In M1b, distant spread has occurred.
Other Factors Determining Treatment Choices and Outcome
Staging factors are used to help determine treatment and outlook. The following suggest a more aggressive disease:
- The presence of respiratory symptoms
- A tumor larger than 3 cm
- High numbers of blood vessels in the tumor
Researchers are always looking for more accurate ways to determine lung cancer treatment and outlook. For example, some research involves specific biomarkers and related blood vessel development within tumors. These markers might eventually help predict the cancer's aggressiveness and determine the best treatment approach.
Using the information, lung cancer is divided into stages, I through IV. Stages I through III, are further divided into A or B (for example stage IA and IB). Each stage will usually have a different approach to treatment.
Treatment Options by Stages
Occult Stage
In the occult stage (TX, N0, M0), cancer cells are found in a sample of a patient's coughed-up sputum, but no cancer cells have yet been detected in the lung.
Treatment Options. Surgically removing the tumor (if one can be located) can allow doctors to identify the stage, and often results in a cure.
Stage 0 or Carcinoma in Situ
Stage 0 or carcinoma in situ (Tis, N0, M0) are noninvasive cancers. Only a few layers of cancer cells are detected within one local area. The cancer has not grown through to the top lining in the lung and can be surgically removed. There is a high risk for development of a second tumor, however.
Treatment Options:
- Surgery, often a limited procedure, where only part of a lobe is removed from the lung.
- In patients who cannot be treated surgically, consider photodynamic therapy, cryotherapy, or brachytherapy (discussed below).
Stage I
In stage I, the cancer has reached the higher layers of the lung but has not spread into the lymph nodes or beyond the lung.
General Treatment Options. The primary treatment is surgery, such as lobectomy, if possible. Patients with poor lung function should have partial lobectomy, if possible. Radiation treatments may be appropriate and beneficial for patients who cannot have surgery. It is not clear if early-stage lung cancer patients who have radiation or chemotherapy in addition to surgery have higher survival rates.
The overall 5-year survival rates for early stage-cancer are around 70% for stage IA and close to 60% for stage IB. Patients should consider smoking cessation programs and clinical trials to prevent cancer from returning after the initial treatment. The risk for recurrence is highest in patients who continue to smoke.
Treatment for stage IA (T1a,bNOMO) and IB (T2aNOMO) lung cancer includes:
- Lobectomy (removal of a whole lobe) or sometimes pneumonectomy (removal of one lung).
- Wedge or segment removal, particularly in patients with poor lung function who cannot handle lobectomy.
- Radiation in selected patients who would not tolerate having surgery or whose cancer is cannot be fully removed.
- In general, chemotherapy is not done following surgery unless the tumor is not completely removed.
Stage II
In stage II the cancer cells have spread to nearby lymph nodes.
General Treatment Options. Surgery, usually removal of a lobe (lobectomy) or one lung (pneumonectomy), is the treatment of choice. Radiation treatment after surgery does not seem to improve survival, but may be performed after an incomplete surgical procedure.
If the tumor is completely removed, radiation therapy is usually not performed after surgery. Patients whose cancer is inoperable may consider radiation and chemotherapy treatments.
Patients who do well after surgical removal of the tumor often receive a platinum-based chemotherapy regimen. Sometimes chemotherapy is considered before surgery as well.
In patients who can complete treatment, 5-year survival rates average around 45% for stage IIA (T2bN0M0;T1a,bN1M0;T2aN1M0) and around 35% for stage IIB (T2bN1M0;T3N0M0).
Stage III
In stage III, the cancer cells have spread beyond the lung to the chest wall, diaphragm, or further lymph nodes, such as those in the neck.
General Treatment Options. Generally, the treatment options for stage III tumors are:
- Surgery, if the tumor and affected lymph nodes can be completely removed.
- Consider chemotherapy or radiation therapy before or after surgery.
- Consider clinical trials using advanced radiation techniques, including continuous hyperfractionated accelerated radiation, or 3-D conformal radiation (discussed below).
- Consider other clinical trials, including those of various combination treatments, preventive radiation therapy to the brain and new drugs.
Combination approaches may be significantly more effective than single treatments.
Stage IIIA (T1a,b,T2a,b,N2M0;T3N1,N2M0;T4N0,N1M0).
Researchers have confirmed good survival rates with resection after chemotherapy and radiation therapy.
Stage IIIB (T4N2M0;anyTN3M0). Some patients may consider surgery if the lymph nodes are not involved (T4, N0), and the tumor can be removed. Surgery may not be an acceptable option for other patients with stage IIIB cancer.
Stage IV
In stage IV (any T, any N, M1), the cancer has spread (metastasized) to other parts of the body.
Treatment Options.
- Combination of two- or three-drug chemotherapies that include platinum-based drugs and newer drugs; the best candidates are patients in otherwise good health, who have a limited number of distant tumors. Chemotherapy is not recommended for patients who are too ill.
- Bevacizumab (Avastin) may be used for patients with non-squamous lung cancer, that has not spread to the brain, and who are not coughing up blood.
- Cetuximab may be used for patients with EGFR mutation.
- EGFR tyrosine kinase inhibitors
- Maintenance chemotherapy
- Endobronchial laser therapy or brachytherapy
- External-beam radiation for symptom relief
- Paclitaxel, gemcitabine, or docetaxel are all additional drug options.
- Other clinical trials
- If metastasized cancer involves only one to four areas in the brain under 4 cm, it may respond to stereotactic radiosurgery (an outpatient procedure without anesthesia) followed by radiation to the brain. Larger tumors are candidates for resection with radiation therapy.
Recurring or Additional New Tumors
Recurring or new tumors occur (usually in the lung again) in half of treated patients. Research shows that a single tumor in the lung is more often a new tumor that, in many cases, may be operable.
Treatment Options.
- Radiation for symptom control
- Chemotherapy with or without bevacizumab (Avastin)
- If the cancer has spread to only one site in the brain, it may respond to surgery, followed by whole-brain radiation. Extended disease-free survival is possible. If the brain tumor is not operable, it is treated with radiation. Even if cancer returns in the brain (in 50% of cases), treating it again is possible in many patients, if the disease has not spread elsewhere in the body.
- Laser therapy or interstitial radiation for tumors inside the airways
- Stereotactic radiosurgery (in a few selected patients)
Surgical Procedures
Surgery is considered in the following circumstances:
- The surgical removal of an entire lobe or parts of a lung is the primary treatment for eligible patients in the early stages of cancer. Recurrence is high after surgery, although the new tumor is often operable.
- Some patients with stage IIIA cancer may also benefit from surgery. The intent at this stage is to extend survival time, rather than cure the disease.
- Surgery is not out of the question in rare cases of metastasis when the cancer appears in a single operable location, such as the brain.
Surgery is often combined with other treatment options. Unfortunately, lung surgery may be too risky for patients with other lung diseases or serious medical conditions, and because lung cancers tend to occur in smokers over 50, such health problems are likely to be present. Long-term survival rates appear to be better in patients treated at hospitals that perform large numbers of lung cancer surgeries, and when surgeries are performed by thoracic surgeons, who specialize in chest procedures.
Newer risk profiles for surgery are being looked at, and patients are participating in decisions about surgery based on the likely risks and benefits.
Standard Surgical Procedures
The type of surgery a patient needs depends on the amount of lung or other tissue that needs to be removed.
Lobectomy. Removal of one of the lobes of the lung is called lobectomy. The patient must have satisfactory lung function to undergo this procedure. The patient has a 3 - 5% risk of death after this operation, with older patients having the highest risk.
Wedge Resection or Segmentectomy. Wedge resection and segmentectomy remove only a small part of the lung. They preserve almost normal breathing function after the operation.
Pneumonectomy. Pneumonectomy removes the entire lung. The patient has a 5 - 8% risk of death after this procedure. The oldest patients have the greatest risk, and they almost always have a recurrence.
These surgeries may be combined with other surgical techniques such as lymphadenectomy and bronchoplasty to repair or remove additional diseased tissue.
Other Procedures
Surgical advances are allowing a wider range of options, including minimal surgeries for early cancers and surgeries that relieve cancer symptoms in the late stages of the disease.
Thoracoscopy. Thoracoscopy, also known as video-assisted thoracic surgery (VATS), is a less-invasive technique that uses a thin tube containing a miniature camera and surgical instruments. It involves much smaller incisions than open surgery (thoracotomy) and speeds recovery to the point that patients are up within hours. Though the procedure is not appropriate in all cases, it offers significant advantages, especially in older or frail patients. The death and complication rates following VATS are lower than those after conventional surgeries. Pain is reduced, and patients are released from the hospital quicker. Several studies found that the 5-year survival and recurrence rates in patients with stage I non-small cell lung cancer treated with VATS were comparable to those in patients treated with traditional open chest surgeries. The number of VATS surgeries has steadily increased every year as physicians gain experience with the technique. Initially it was primarily used in wedge resections, but is now used for lobectomies and segmentectomies as well.
Laser Surgery. Laser surgery allows surgeons to remove small amounts of lung tissue, and it is proving useful for improving symptoms in stage II and IIIA patients. Laser surgery may also be beneficial in treating cancers that have spread to, and are obstructing, the throat.
Photodynamic Therapy. Photodynamic therapy uses bronchoscopy and special laser light beams combined with a light-sensitive drug, called porfimer sodium (Photofrin), to kill cancer cells. The most common side effect is sun sensitivity. Bleeding in the lungs is a more serious side effect. Photodynamic therapy may be considered for patients in early-stage disease who are not candidates for other surgical procedures. It may also be used to reduce symptoms in late-stage disease.
Cryosurgery. Cryosurgery uses a probe chilled to below freezing to destroy the tumor cells on contact. It is being investigated in combination with radiation therapy. It may also be an alternative in early stage cancer for patients who cannot have surgery.
Electric Cauterization and Thermal Ablation. Electric cauterization, which uses electricity to produce heat that destroys tissue, is also under investigation as a treatment for early-stage disease.
Radiofrequency Ablation. This non-surgical technique that uses an x-ray guided electrode to deliver heat to tissues may benefit lung cancer patients who do not accept surgery, or are not eligible for surgery, radiation, or chemotherapy. In one study, 70% of patients treated with this method survived for at least one year. Because the technique spares damage to nearby tissues, patients tend to have minimal side effects. More research is needed to confirm the benefit of radiofrequency ablation over other, non-surgical treatment options.
Radiation Treatments
In addition to surgery, radiation is the other primary treatment for early-stage lung cancer. Doctors are also studying the benefits of radiation treatment in advanced lung cancer.
Radical Radiation in Early-Stage Cancer. Radical radiation is used as the sole procedure in stage I and some stage II patients who have adequate lung function but, for medical or other reasons, cannot be treated with surgery. Chest
radiation therapy such as conventional radiotherapy or stereotactic body radiation therapy may be performed.
Combined Treatments for Improving Survival in Advanced Cancer. Radiation is also being investigated in various combinations with chemotherapy, surgery, or both. Radiation treatment plus platinum-based chemotherapy may extend survival times in advanced lung cancer. Other combinations are also showing promise.
Palliative Radiation. Doctors use palliative radiation to shrink tumors and reduce pain and symptoms. Palliative radiation is appropriate for patients with advanced disease and poor lung function, or for those with cancer that has spread. In up to 85% of patients with advanced disease, palliative radiation therapy helps relieve pain, shortness of breath, superior vena cava syndrome, coughing up blood, and symptoms caused by cancer that has spread to the brain. Radiation in these cases is not generally used to reduce mortality rates, although it may increase survival in some patients, such as those with excellent lung function whose tumors are small.
Delaying radiation therapy until symptoms develop in patients with minimal or no symptoms does not appear to reduce survival times or impair quality of life compared to starting it right away.
Radiation Therapy in Metastasis to the Brain. Radiation is the primary treatment when cancer has spread to the brain, unless the cancer is small enough to be treated surgically. When radiation is used, a technique called stereotactic radiosurgery may deliver powerful, highly targeted radiation to specific areas in the brain. The procedure takes about 30 minutes to one hour and patients typically go home the same day. Up to five sessions may be performed.
Some trials are investigating the benefits of radiation to the head to prevent the cancer from damaging the brain.
Standard Radiation Procedures
The goal of radiation treatment is to administer doses as high as possible to kill as many cancer cells as possible, without destroying surrounding healthy tissues or causing a dangerous reaction. Doctors may try different procedures for the same patient. The exact radiation procedure depends on the site of the cancer or how far it has spread.
- External-Beam Radiation. External-beam radiation therapy focuses a beam of radiation directly on the tumor. It is generally used for cancer that has spread.
- Brachytherapy. Brachytherapy implants radioactive seeds through thin tubes directly into the cancer sites. Brachytherapy may be used for lung cancers that have spread to the throat and caused obstruction. High-dose-rate brachytherapy may also have some value for patients with inoperable tumors in the central region of the lung.
Hyperfractionated Radiotherapy
Hyperfractionated radiotherapy gives smaller-than-standard doses a number of times a day (usually two or three). This allows doctors to use a higher dose over the whole course of treatment. It is not as useful as therapy by itself, but can have survival benefits when combined with chemotherapy.
Continuous Hyperfractionated Accelerated Radiotherapy. Continuous hyperfractionated accelerated radiotherapy (CHART) administers multiple doses of radiation per day but uses the standard doses. This allows the total dose of radiation to be administered over a shorter time period than the standard 6 weeks. CHART may give patients with localized cancer better survival rates than standard radiotherapy or non-accelerated hyperfractionated radiation. It can cause severe swallowing problems, though. Modifying the treatment by stopping it for 2 days out of 7 may help reduce this effect.
Three-Dimensional Conformal Radiotherapy
Three-dimensional (3-D) conformal radiotherapy delivers external-beam radiation specifically to targeted organs or tissues. This allows doctors to administer significantly higher doses to attack the cancer, while reducing the risk to healthy cells. This technique is generally considered the standard method of delivering radiation to lung tumors.
Side Effects of Radiation Therapy
Radiation can have significant side effects when used as part of intensive treatments, such as hyperfractionated radiotherapy or radiotherapy in combination with chemotherapy. Among the most serious problems is severe inflammation in the esophagus (esophagitis) or lungs (pneumonitis). Infection is also a danger.
The use of targeted approaches, such as conformal radiotherapy, may help reduce these complications.
Chemotherapy Treatments
Chemotherapy is the use of drugs given by mouth or injection to destroy cancer cells that may have spread beyond the tumor. Until recently, there has been some doubt about the effectiveness of chemotherapy for lung cancer. A major analysis of 52 trials supported its use, particularly with platinum-based regimens, and with the combination of supportive care and sometimes surgery. Chemotherapy can offer an improvement in survival for several stages of advanced lung cancer.
- Chemotherapy in early stages: Chemotherapy is proving to be beneficial in many patients as an additional (adjuvant) treatment with surgery or radiation.
- Chemotherapy in advanced disease: Chemotherapy may be used as first-line treatment in patients with inoperable or metastasized lung cancer. It is typically used in late stages to reduce symptoms and, in some cases, extend survival.
Chemotherapy Drugs and Regimens
Most chemotherapy regimens use platinum compounds, either cisplatin (Platinol) or carboplatin (Paraplatin). The preferred regimen uses two drugs -- one of which is a platinum-based drug. Combinations may include third generation drugs such as paclitaxel (Taxol) and carboplatin or cisplatin. This regimen can also include gemcitabine, docetaxel, vinblastine (vindesine or vinorelbine), irinotecan, or pemetrexed. The gemcitabine and vinorelbine combination might be a good option for patients who cannot tolerate platinum compounds. Researchers are looking at genetic mutations, often referred to as biologic markers, and the effectiveness of customized chemotherapy options for specific mutations.
Pemetrexed (Alimta). Pemetrexed, known as an anti-folate, is a newer chemotherapy drug for first-line treatment of advanced nonsquamous non-small cell lung cancer, in combination with cisplatin. The drug targets a number of enzymes that play a role in how cancer cells increase. Pemetrexed does have some serious toxic effects, but they can be significantly reduced with folic acid and vitamin B12 supplements.
Attention is also being given to agents called biologic response modifiers, such as the EGFR gene inhibitor gefitinib (Iressa). Gefitinib (Iressa), a second-line therapy for non-small cell lung cancer, is available for a limited group of patients. Gefitinib (Iressa) may also be recommended as first-line therapy in select patients with advanced disease with EGFR mutation.
Erlotinib (Tarceva) is in the same medication class as gefitinib. It is approved for patients with locally advanced or metastatic non-small cell lung cancer who have failed one type of chemotherapy treatment in the past (it is a second-line treatment). It is also prescribed as a maintenance treatment when cancer has not spread or grown after chemotherapy treatment. Erlotinib plus targeted therapy shows survival and progression free benefits compared to placebo. No studies comparing erlotinib to gefinitib have been conducted and further research is necessary to assess the overall survival benefits of both drugs. Both are taken orally, once daily.
Another agent, bevacizumab (Avastin) is combined with platinum-based chemotherapy as a first-line treatment choice for patients with advanced, non-squamous cancer. Bevacizumab is a monoclonal antibody (MAb) which inhibits growth of new blood vessels. Studies show that it increases survival, but unfortunately only for less than 2 months.
In select advanced lung cancer patients with EGFR mutation, cetuximab (Erbitux), a monoclonal antibody approved in 2012, may be added to a cisplatin/vinorelbine regimen as first-line therapy.
Administration, Timing, and Drug Sequences
Chemotherapy treatments are usually performed in an outpatient setting. They are given in regular cycles for several months. Researchers are still investigating how many chemotherapy cycles to administer in late-stage cancers, the timing of those cycles, and the sequences of the drugs. For instance, a three- or four-course cycle may achieve the same survival times and better quality of life than the standard of six or more course cycles. However, changing even one day in a drug sequence can sometimes significantly affect the outcome. Such fine-tuning of chemotherapy regimens is likely to have the most effect on patients with advanced-stage disease, which requires more tailored treatment than early-stage disease.
Side Effects
Side effects of chemotherapy treatments are common, and they are more severe with higher doses. Side effects increase over the course of treatment. Some studies suggest that side effects can be reduced by giving the drugs for shorter durations, without losing the cancer-killing effects.
Common side effects include the following:
- Diarrhea
- Temporary hair loss
- Weight loss
- Fatigue
- Depression
- Nausea and vomiting: Drugs known as serotonin antagonists, especially ondansetron (Zofran), can relieve these two side effects. Serotonin antagonists work well in nearly all patients given moderate chemotherapy drugs, and in most patients who take more powerful drugs.
- Anemia: An abnormally low number of red blood cells is common in lung cancer. One treatment involves transfusions or injections of erythropoietin, a drug that increases red blood cell production. Erythropoietin is available as epoetin alfa (Epogen, Procrit) and darbepoetin alfa (Aranesp), which requires fewer injections. These drugs are recommended when a patient's hemoglobin level falls below a certain level, usually less than 10 g/dL.
These side effects are nearly always temporary. Most patients are able to continue with their normal activities for all but perhaps 1 or 2 days per month.
Serious complications of chemotherapy can also occur, and vary depending on the specific drugs. These complications include:
- Increased chance for infection from suppression of the immune system.
- Severe drop in white blood cells (neutropenia): Certain chemotherapy drugs, such as taxanes, pose a higher risk for this complication than other drugs. A drug called granulocyte colony-stimulating factor (filgrastim and lenograstim) can improve the white blood cell count.
- Liver and kidney damage: Amifostine (Ethyol) reduces the risk for kidney damage in patients taking repeated regimens of cisplatin-based therapy. It is also a radioprotector; that is, it helps prevent severe effects in the esophagus from radiotherapy, with or without chemotherapy.
- Abnormal blood clotting (thrombocytopenia).
- Allergic reaction, particularly to platinum-based agents: A simple skin test is under investigation that may identify people with a potential allergic response.
Second-Line Chemotherapy
Second-line chemotherapy is used for patients whose cancers have come back after the first round of chemotherapy. Several of these agents listed below have prolonged survival for patients with non-small cell lung cancer. Unfortunately, this survival benefit is usually only a matter of several months. Efforts are under way to identify which patients are more likely to benefit from these therapies. Because platinum-based agents are most often used first, they are not beneficial for second-line therapy.
Commonly used second-line agents include:
- Docetaxel (Taxotere). Docetaxel is the drug of choice at this time for cancers that do not respond to initial chemotherapy. Studies have reported that it achieves longer survival times than supportive care alone. It is usually given every 21 days. This regimen causes more side effects than pemetrexed, the newer second-line drug. Weekly doses of docetaxel are effective and less toxic than the 3-week schedule. It is not clear if the weekly schedule achieves survival rates comparable to those of pemetrexed, however.
- Pemetrexed (Alimta). Pemetrexed, a first-line treatment of nonsquamous non-small cell lung cancer in combination with cisplatin, is also approved as a single second-line treatment of the same type of cancer. Some research suggests that it is as effective as docetaxel. It is less toxic than docetaxel when docetaxel is given every 21 days, but not when it is given weekly.
- Erlotinib (Tarceva), Gefitinib (Iressa) and Other Tyrosine Kinase Inhibitors. Research is focusing on drugs that block small molecules involved with the growth of blood vessels that feed the tumor (a process called angiogenesis). Compounds called growth factors, which may be important in cancer cell production, control the growth of these new blood vessels. Medications that literally turn off these growth factors or their receptors may be able to cut off cancer's lifeblood. Gefitinib and erlotinib are angiogenesis inhibitors.
- Erlotinib (Tarceva) was approved as a single agent second-line therapy in November 2004. Erlotinib is taken by mouth and has very low risk of side effects (rash and diarrhea are common). Erlotinib was more recently approved as first-line therapy for patients with locally advanced NSCLC. Select patients with EGFR gene involvement are most likely to benefit from first-line therapy with erlotinib.
- Gefitinib (Iressa) has limited approval as a second-line therapy for non-small cell lung cancer. It has shown promise in patients with EGFR gene involvement.
- Erlotinib may also be considered as third-line therapy for advanced lung cancer patients whom have not yet tried erlotinib or gefinitib.
Combinations of Chemotherapy with Surgery, Radiation Therapy, or Both
Particularly for more aggressive or advanced cancers, different combinations of surgery, chemotherapy, and radiation therapy may be tried. These include:
- Chemotherapy Following Surgery (Adjuvant Chemotherapy). Evidence is now supporting the use of platinum-based chemotherapy after surgery in some patients with lung cancers. Not all studies confirm survival benefits, however, and trials are ongoing.
- Chemotherapy before Surgery (Induction or neoadjuvant Chemotherapy). Induction chemotherapy may be used to shrink tumors before surgery. Studies have been mixed as to whether there are any survival benefits in patients with advanced lung cancer.
- Combined and Multi-Modal Therapy. In more advanced cancers, investigators are researching very intensive treatments that use two or more combinations of chemotherapy, radiation, and surgery. For example, radiation plus chemotherapy may be helpful in patients whose tumors are surgically removable. Such approaches are very toxic but appear to improve survival in selected patients.
Severe inflammation in the esophagus is the most common severe side effect of the radiation and chemotherapy combination. There is also a very high risk of serious infections, including pneumonia, herpes zoster, and cytomegalovirus. Long-term antibiotic therapy may be needed.
Although patients over 70 may suffer more from toxic effects than younger patients, studies now suggest that they can achieve survival rates with combined treatments that are equal to those in younger patients.
Agents Used for Pain Relief
There are many painkilling medications available. Research shows that aggressive pain relief can help patients better manage cancer treatment symptoms. For example, reducing pain in elderly cancer patients may markedly lower their fatigue levels, and improve other symptoms as well.
Opioids are the most potent painkillers. The correct use of these strong medications is very important for reaching acceptable pain relief and preventing a toxic response. For example, the long-lasting version of oxycodone (OxyContin) must be swallowed whole. Chewing, inhaling, or injecting it can create a deadly overdose.
Investigative Agents
According to a 2001 article, of the nearly 500 cancer drugs in development, 58 (about 13%) were aimed at fighting lung cancer. Only breast cancer had a higher percentage of new drugs in development. Unfortunately, no drugs to date have shown any real benefit in terms of patient survival. However, some drugs are showing promise, and at this time, these agents are the best hope for improving lung cancer survival rates.
Monoclonal Antibodies (MAbs)
Monoclonal antibodies (MAbs) are genetically designed immune factors. MAbs mark foreign compounds called antigens for attack by the immune system. Bevacizumab (Avastin) was approved in October 2006 as a first-line treatment (in combination with carboplatin and paclitaxel) for inoperable, locally advanced, metastatic, or recurrent non-squamous, non-small cell lung cancer. Cetuximab (Erbitux) was recently approved for recurrent local disease or metastatic squamous cell cancer of the head and neck. It is considered in patients with EGFR mutation. Trastuzumab (Herceptin) is also under investigation for the treatment of lung cancer.
All of these MAbs block epidermal growth factor. These drugs are of particular interest for patients who have cancers that produce too much of the protein called HER2. They show great promise in combination with chemotherapies and newer drugs, such as the tyrosine kinase inhibitors. For example, adding bevacizumab to platinum-based chemotherapy extends the disease-free survival time in patients with advanced non-small cell lung cancer.
Resources
- www.cancer.gov -- National Cancer Institute
- www.cancer.org -- American Cancer Society
- www.cancercare.org -- Cancer Care
- www.lungusa.org -- The American Lung Association
- www.asco.org -- American Society of Clinical Oncology
- www.alcase.org -- Alliance for Lung Cancer
- www.lungcancer.org -- Joint project of Cancer Care and the Oncology Nursing Society
- www.nccn.org -- National Comprehensive Cancer Network
- www.lungcanceronline.org -- Lung cancer information
- www.epa.gov/radon/ -- National radon information
- www.clinicaltrials.gov -- Find clinical trials
- www.cancer.gov/clinicaltrials -- Find clinical trials
References
Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379-86.
Alberg AJ, Ford JG, Samet JM; American College of Chest Physicians. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:29S-55S.
Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304(20):2245-52.
Azzoli C, Temin S, Alif T, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non–Small-Cell Lung Cancer. JCO. 2011; vol. 29(28):3825-3831.
Bach PB, Silvestri GA, Hanger M, Jett JR. Screening for lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:69S-77S.
Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364(10):947-55. Review.
Chlebowski RT, Schwartz AG, Wakelee H, et al; Women's Health Initiative Investigators. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374(9697):1243-51.
Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432-40.
Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136(1):260-71. Review.
Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361(1):32-39.
Gettinger S, Lynch T. A decade of advances in treatment for advanced non-smallcell lung cancer. Clin Chest Med. 2011;32(4):839-51. Review.
Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet. 2011;378(9804):1727-40.
Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367-80. Review.
Jett JR, Schild SE, Keith RL, Kesler KA. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:266S-276S.
Johnson DH, Blot WJ, Carbone DP, et al. Cancer of the lung: Non-small cell lung cancer and small cell lung cancer. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKena WG. Clinical Oncology. 4th ed. Philadelphia, Pa: Churchill Livingstone Elsevier; 2008:chap 76.
Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621-628.
Lilly Inc. Alimta Prescribing Information. Rev. 10/2008.
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-8.
Maziak DE, Darling GE, Inculet RI, et al. Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med. 2009;151(4):221-8,W-48.
Mehra R, Moore BA, Crothers K, Tetrault J, Fiellin DA. The association between marijuana smoking and lung cancer: a systematic review. Arch Intern Med. 2006 Jul 10;166(13):1359-67.
Mehta HJ, Ross C, Silvestri GA, Decker RH. Evaluation and treatment of high-risk patients with early-stage lung cancer. Clin Chest Med. 2011;32(4):783-97. Review.
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-57.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008; 83(5):584-594.
National Cancer Institute. Lung Cancer Home Page. Bethesda, Md.: U.S. National Institutes of Health. Accessed August 3, 2008.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 2.2008. Accessed July 3, 2009.
National Lung Cancer Trial Research Team. Reduced Lung-Cancer Mortality with Low-dose Computed Tomographic Screening. N Eng J Med. 2011.
Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2010 May 12;(5):CD007309. Review.
NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operablenonsmall-cell lung cancer: two meta-analyses of individual patient data. Lancet.2010;375(9722):1267-77.
Paoletti L, Pastis NJ, Denlinger CE, Silvestri GA. A decade of advances in treatment of early-stage lung cancer. Clin Chest Med. 2011;32(4):827-38. Review.
Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. Review.
Pirker R, Pereira JR, Szczesna A, et al; FLEX Study Team. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525-31.
Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:131S-148S.
Robinson LA, Ruckdeschel J, Wagner H, Stevens CW. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:243S-265S.
Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:234S-242S.
Shen KR, Meyers BF, Larner JM, Jones DR. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:290S-305S.
Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, Detterbeck F. Noninvasive staging of non-small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:178S-201S.
Silvestri GA, Jett J. Clinical Aspects of Lung Cancer. In: Mason and Nadel's Textbook of Respiratory Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier; 2010:chap 47.
Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long-term use of supplemental vitamins, vitamin C, Vitamin E, and folate does not reduce the risk of lung cancer. Am J Respir Crit Care Med. 2008;177:524-530.
Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362(12):1119-27. Review.
Tassinari D, Scarpi E, Sartori S, et al. Second-line treatments in non-small cell lung cancer. A systematic review of literature and metaanalysis of randomized clinical trials. Chest. 2009;135(6):1596-1609.
Temel JS,Greer J, Muzikansky J, et al. Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med. 2010; 363(8): 733-42.
U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006.
Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med. 2008;168:1097-1103.
|
Review Date:
9/14/2012 Reviewed By: Harvey Simon, MD, Editor-in-Chief, Associate Professor of Medicine, Harvard Medical School; Physician, Massachusetts General Hospital. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M. Health Solutions, Ebix, Inc. |